ByHeart Infant Formula Recalled Due to Potential Contamination
Five lots of formula are being recalled after a sample tested positive for Cronobacter sakazakii, rare but serious bacteria

Update: This story has been updated to reflect that per the Food and Drug Administration, the ByHeart recall affected 30,000 cans (not 30,000 people.)
The company ByHeart has recalled five lots of infant formula because they could be contaminated with Cronobacter sakazakii, rare but serious and sometimes deadly bacteria. No illnesses have been reported.
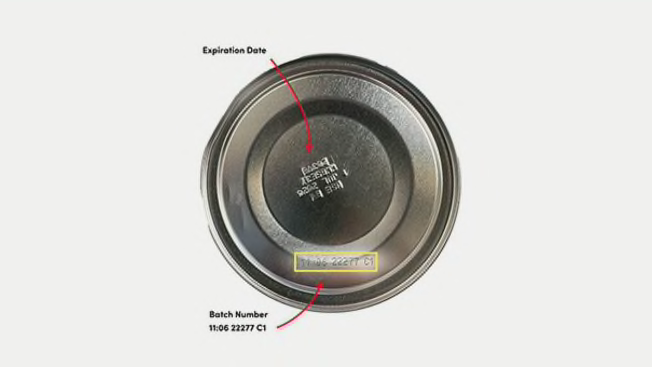
The product being recalled is called ByHeart Whole Nutrition Infant Formula, Milk Based Powder with Iron for 0-12 Months. It’s packaged in 24-ounce containers that will have one of these codes on the bottom of the can: 22273 C1, 22276 C1, 22277 C1, 22278 C1, or 22280 C1, and a use-by date of “01 JAN 24” or “01JUL 24.”
The company told CR in a statement that Cronobacter was detected in a sample collected from a third-party facility that packages the formula for ByHeart. All products packaged on that day, and from the first production on the next day, were destroyed and were not distributed, the company said.
Risk of Cronobacter
Cronobacter is a relatively rare infection, according to the Centers for Disease Control and Prevention. At greatest risk are infants 2 months or younger and those who may have weakened immune systems from being sick with other conditions or receiving medical treatment.
Symptoms include fever, poor feeding, crying, or very low energy, according to the Centers for Disease Control and Prevention. In more advanced cases, the bacteria can cause a blood infection and sepsis, as well as swelling around the linings of the brain and spinal cord, also known as meningitis.
Source: FDA Source: FDA
The Details
Products recalled: ByHeart Whole Nutrition Infant Formula, Milk Based Powder with Iron for 0-12 Months in 24-ounce containers that are marked on the bottom with one of these numbers: 22273 C1, 22276 C1, 22277 C1, 22278 C1, or 22280 C1, marked with use-by dates of 01 JAN 24 or 01JUL 24.
The problem: The infant formula may be contaminated with Cronobacter.
The fix: Throw it out. Customers who purchased the recalled formula should receive an email from ByHeart saying it will offer two additional cans of formula in coming shipments.
How to contact the manufacturer: Consumers with questions can email the company at notices@byheart.com or send a text to 909-506-2354. The company has also published an FAQ about the recall.
Correction: This article, which originally published on Dec. 15, 2022, should have said that about 30,000 cans had been distributed to customers.